Chemical Bonding and Molecular Structure:
- Admin
- Oct 25, 2022
- 9 min read
The observation of chemical links among atoms or molecules is referred to as chemical bonding. This chapter discusses why the simplest unique atoms can combine to shape a brand-new product and why they should be organized in a particular shape. VSEPR, valence bond theory and molecular orbital theory are a number of theories that may provide an explanation for all the occurrences in detail. Bonding is not without a doubt an example; it is nature's manner of bringing each atom or molecule to the maximum solid-state possible.
Every shape withinside the universe is the outcome of the development of unique types of bonds. Bonding is nothing greater than the joining of atoms.
Kossel – Lewis Approach to Chemical Bonding
A chemical bond is an attractive force between atoms in a molecule.
In 1916, Cossel and Lewis succeeded in explaining chemical bonds in terms of electrons.
Octet Rule: Like noble gas atoms, atoms of various elements try to achieve electronic configurations or complete octets by chemical bonding. In other words, the atoms of all main group elements tend to bond such that each atom has eight electrons in its valence shell, allowing the atoms to achieve noble gas-like electronic configurations. Atoms are stabilized like noble gases by chemical bonds.
Lewis postulated that atoms achieve stable octets when joined by chemical bonds.
Lewis symbol – Only valence electrons (electrons in the outermost shell of atoms) participate in chemical bonds when a molecule is formed. American chemist G.N. Lewis introduced a simple notation to represent the valence electrons of atoms. These notations are known as Lewis symbols.

Electrovalent Bonds – Bonds formed because of electrostatic attraction between positive and negative ions are called electrovalent bonds. Also called an ionic bond.
The number of electrons lost or gained during the formation of electronic bonds is called the valence of the element.

Factors Which Affect the Formation of Ionic Bond:
Enthalpy of Ionization: The enthalpy of ionization of an element is the amount of energy required to remove an electron from the outermost shell of an isolated atom in the gas phase and convert it to a gaseous positive ion. Also called ionization energy. The lower the ionization enthalpy, the easier it is to remove electrons. H. The formation of cations, and a higher probability of ionic bond formation.
Electron Gain Enthalpy: The electron affinity or electron gain enthalpy of an element is the change in enthalpy that occurs when an additional electron is added to an isolated atom in the gas phase to form a gaseous anion.
The higher the electron affinity, the more energy is released and the more stable the negative ions produced. As a result, the probability of ionic bond formation increases.
Lattice Energy: The energy released when the required number of gaseous cations and anions combine to form one mole of ionic compound is called the lattice enthalpy
Characteristics of Ionic Compounds:
Ionic compounds usually exist in a solid state.
In ionic compounds, the ions are arranged in regular patterns. Therefore, they have a specific crystal structure. For example, NaCl has an octahedral crystal structure.
Ionic compounds usually have high melting and boiling points. This is because the ions are held together in ionic compounds by strong electrostatic forces.
Ionic compounds are generally soluble in polar solvents such as water.
Solutions of ionic compounds are good conductors. It is a good electrical conductor even in a molten state.
Ionic compounds are involved in a variety of chemical reactions.
Covalent Bonds: Bonds created by sharing electrons between atoms are called covalent bonds. This term and idea were introduced by Langmuir in 1919.

The dots in the structure above represent valence electrons (Lewis symbols). Such structures are called Lewis point structures. Two atoms can have single, double, or triple covalent bonds.
Formal Charge: The formal charge of an atom in a polyatomic molecule can be defined as the difference between the number of valence electrons of the atom in the isolated or free state and the number of electrons assigned to that atom in the Lewis structure. It helps to select the most stable structure from various possible Lewis structures. H. The one with the lowest energy.
Atom's formal charge in the Lewis structure = total number of free atom valence electrons - total number of non-bonded electrons - 1/2 total number of shared electrons.
Example: Formal charge on atoms in carbonate ions.

Lewis Structure of CO3-2 Ion:

Formal charge on C atom = 4 – 0 - ½ (8) = 0
Formal charge on double bonded O atom = 6 – 4 - ½ (4) = 0
Formal charge on single bonded O atom = 6 – 6 - ½ (2) = -1
Bond Parameters:
Factors Affecting Bond Length
Bond Length: The bond period is described because of the equilibrium distance among the nuclei of bonded atoms in a molecule.
Atom Size: Bond lengths increase with increasing atom size. For example, the order of bond lengths for H-X is:
HI > HBr > HCl > HF
Bond Multiplicity: Bond length decreases with bond multiplicity. Therefore, the bond length of the carbon-carbon bond is on the order of
C ≡ C < C > sp2 C-H > sp C-H
s characters - (25%) (33%) (50%).
Bond Angles:
Bond angles are defined as the angles between orbitals containing a bond electron pair around the central atom of a molecule or complex ion. This can be determined experimentally using spectroscopy. Expressed in degrees.
Bond Enthalpy: Bond enthalpy is defined as the amount of energy required to break one mole of a bond of a particular type between two atoms in the gaseous state.
Factors Affecting Bond Energy –Atom Size - The larger the atom, the longer the bond length and the lower the bond dissociation enthalpy. H. Adhesive strength is low.
Bond Multiplicity: For bonds between the same two atoms, the greater the bond multiplicity, the greater the bond dissociation enthalpy. First, this is because the atoms are closer together, and second because the number of bonds that are broken is higher. is the order. ○=○< N N
Number of lone pairs present: The greater the number of lone pairs present in the bond atoms, the greater the repulsive force between atoms, and the lower the bond dissociation enthalpy.
Bond Order: In Lewis' description of covalent bonds, bond order is given by the number of bonds between two atoms in a molecule.
Isoelectronic molecules and ions have the same bonding order. The bond order between F2 and O22- is 1 and is isoelectronic.
As bond order increases, bond enthalpy increases and bond length decreases. So we can write -
bond order ∝ bond enthalpy ∝ 1 bond length
Dipole Moment: The dipole moment can be defined as the product of the magnitude of the charge and the distance between the centers of the positive and negative charges. It is represented by the Greek letter "μ". Mathematically it can be expressed as -
dipole moment = charge x separation distance
μ = Q x d
expressed in Debye units (D). 1D = 3.33564 10-30 C m (C = Coulomb, m = meter)
Vector quantity. This is represented by a small arrow with a tail in the positive center and a head towards the negative center. For example, the dipole moment of HCl is expressed as

Importance of dipole moment: zero-moment molecules are non-polar molecules and those with μnet ≠ 0 are inherently polar.
Dipole moment values can be used to determine the ionic properties of a bond. The formula for determining percent ion characteristics is given below
Percentage of ionic character =
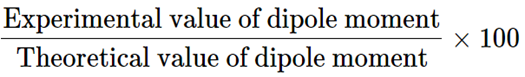
The Valence Shell Electron Pair Repulsion (VSEPR) Theory:
The valence shell electron pair repulsion theory explains the shape of the molecule. It is based on repulsive interactions between electron pairs in the valence shells of atoms. This theory was advanced by Sidgwick and Powell in his 1940 and further improved by Nyholm and Gillespie in 1957. The main assumptions of VSEPR theory are:
is unconstrained around the central atom.
The electron pairs in the valence shell repel each other because the electron cloud is negatively charged.
These electron pairs tend to occupy positions in space that minimize repulsion, thus maximizing the distance between electron pairs.
The valence shell is assumed to be a sphere, and electron pairs on the surface of the sphere are at maximum distance from each other.
Multiple bonds are treated as if they were lone pairs, and two or three electron pairs of multiple bonds are treated as lone super pairs.
If more than one resonance structure can describe a molecule, the VSEPR model can be applied to such structures.
Decreasing Order of the Repulsive Interaction of Electron Pairs:
Lone pair – lone pair > lone pair-bond pair > Bond pair – Bond pair
Or
lp – lp > lp – bp > bp – bp
Valence Bond Theory:
The valence bond theory was proposed by Heitler and London in 1927 and further developed by Pauling and others. It is based on the electronic configuration of elements, atomic orbital duplication criteria, atomic orbital hybridization, and the principles of variation and superposition. In Class XI Chemistry, valence bond theory is treated at a fundamental level only on a qualitative level, not in mathematical terms.
Attraction occurs between the nucleus and its own electrons.
The nucleus of one atom and the electrons of another.
electrons of two atoms
Repulsion occurs between
The nuclei of two atoms.
Eventually, the net attractive force balances the repulsive force and the molecule gains minimal energy and stabilizes.
Sigma bond: The strongest covalent bond formed by heads on overlapping atomic orbitals is called the sigma bond. Sigma bonds are found in alkanes, alkenes and alkynes. Formed by s-s overlap, sp overlap and p-p overlap. The formation of a sigma bond is shown below between orbitals

Pi Bond: A covalent bond formed by the lateral overlap of half-filled atomic orbitals of an atom is called a pi bond. Alkenes and alkynes have a pi bond. The formation of a pi bond is shown below between two orbitals.
Hybridization: The concept of hybridization was introduced by Pauling. Atomic orbitals combine to form a new set of equivalent orbitals known as hybrid orbitals. This phenomenon is known as hybridization. These hybrid orbitals are involved in bond formation.
The main characteristics of hybrid orbitals are:
number of hybrid orbitals = number of atomic orbitals participating in a hybrid orbital
hybrid orbitals are always equivalent in energy and shape.
Hybrid orbitals are more efficient at forming stronger bonds than pure atomic orbitals.
These hybrid orbitals are favourably oriented in space to minimize repulsion between electron pairs. thus, forming a stable arrangement.
Hybridization type indicates the shape of the molecule.
Hybridization Conditions - Orbitals in the valence shell of the atom hybridize.
hybrid orbitals should have approximately the same energy.
No electron boost is required prior to hybridization.
It is not necessary that only half-filled orbitals can participate in hybridization. The filled orbitals of the valence shell also participate in hybridization.
Types of hybridization:
hybridization occurs by including various orbitals such as s, p, and d orbitals. It is of the following type:
sp hybridization: one’s orbital and one p orbital participates in the hybridization and each sp hybrid orbital has 50% s and 50% p characters. Two sp hybrid orbitals are formed.
sp2 hybridization: 1 s and 2 p orbitals participate in this hybridization to form 3 hybrid orbitals. Each of these hybrid orbitals exhibits s-characteristics of 33% and p-characteristics of 67%.
sp3 hybridization: 1 s orbital and 3 p orbitals participate in this hybridization to form 4 hybrid orbitals. Each of these hybrid orbitals exhibits 25% s-characteristics and 75% p-characteristics.
dsp2 hybridization: 1 s, 2 p, and 1 d orbitals participate in this hybridization to form 4 hybrid orbitals.
sp3d hybridization: 1 s, 3 p, and 1 d orbitals participate in this hybridization to form 5 hybrid orbitals.
sp3d2 hybridization; 1 s, 3 p, and 2 d orbitals participate in this hybridization to form 3 hybrid orbitals.
d2sp3 hybridization: 1 s, 3 p, and 2 d orbitals participate in this hybridization to form 3 hybrid orbitals.

Molecular Orbital Theory:
The molecular orbital theory is another approach to explaining chemical bonding in molecules. Built-in 1932 by Mulliken and Hund. The molecular orbital theory considers the whole molecule as a unit in which all electrons move under the influence of all nuclei present in the molecule. The salient features of the molecular orbital theory are:
Just like atomic orbitals lie around the nucleus of an atom, there are molecular orbitals that lie around the nucleus of molecules.
Molecular orbitals are quite different from the atomic orbitals from which they are formed. Atomic orbitals fuse to form molecular orbitals. Conditions for Atomic Orbitals to Form Molecular Orbitals -
Bonding atomic orbitals must be of equal energy.
Connecting atomic orbitals must overlap significantly. The greater the overlap, the more stable the molecular shape.
The valence electrons of the constituent atoms are thought to move along the molecular orbital under the influence of the nucleus contained.
Molecular orbitals have different energy levels like the atomic orbitals of an isolated atom.
The shape of molecular orbitals depends on the shape of the atomic orbitals from which they are formed.
Like atomic orbitals, molecular orbitals are ordered in descending order of energy.
The number of molecular orbitals formed is the same as the number of atomic orbitals that combine to form bonds.
Similar to atomic orbitals, electron occupancy in molecular orbitals follows three principles: the structuring principle, Hund's law, and Paul's exclusion principle.
Hydrogen Bonds:
Hydrogen bonds can be defined as the attractive force that binds the hydrogen atoms of one molecule to the electronegative atoms of another molecule. Also called hydrogen bonding. It's a very weak bond.

Hydrogen Bonding Conditions: Molecules must contain strongly electronegative atoms such as F, Cl, Br, etc., and electronegative atoms must be small in size.
Types of Hydrogen Bonds:
There are two types:
Intermolecular Hydrogen Bonds: When hydrogen bonds occur between different molecules of the same or different compounds, they are called intermolecular hydrogen bonds.
Example: Hydroalcohol.
Intramolecular Hydrogen Bonds: Hydrogen bonds that occur within the molecule itself. A bond is formed between a hat atom in one group and a highly electronegative atom in the other group.
Example: hydrogen bonding of o-nitrophenol molecules.





Comments